Pipeline
We are focused on the discovery and development of potentially best-in-class or first-in-class small molecule therapeutics. To achieve this, we aim to address issues such as tolerability and combinability, resistance, and disease escape through brain metastases.
Our lead product candidates include:
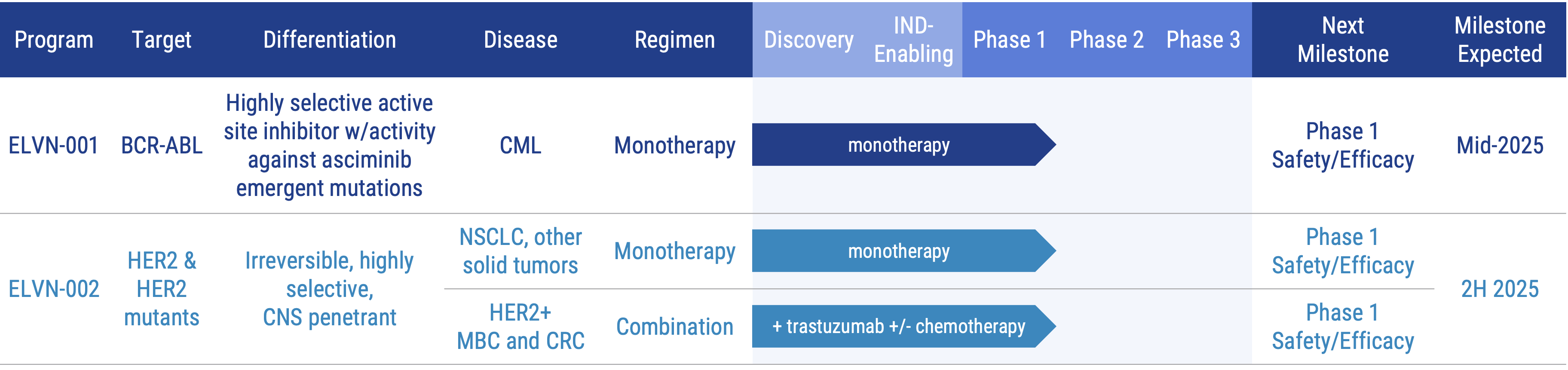
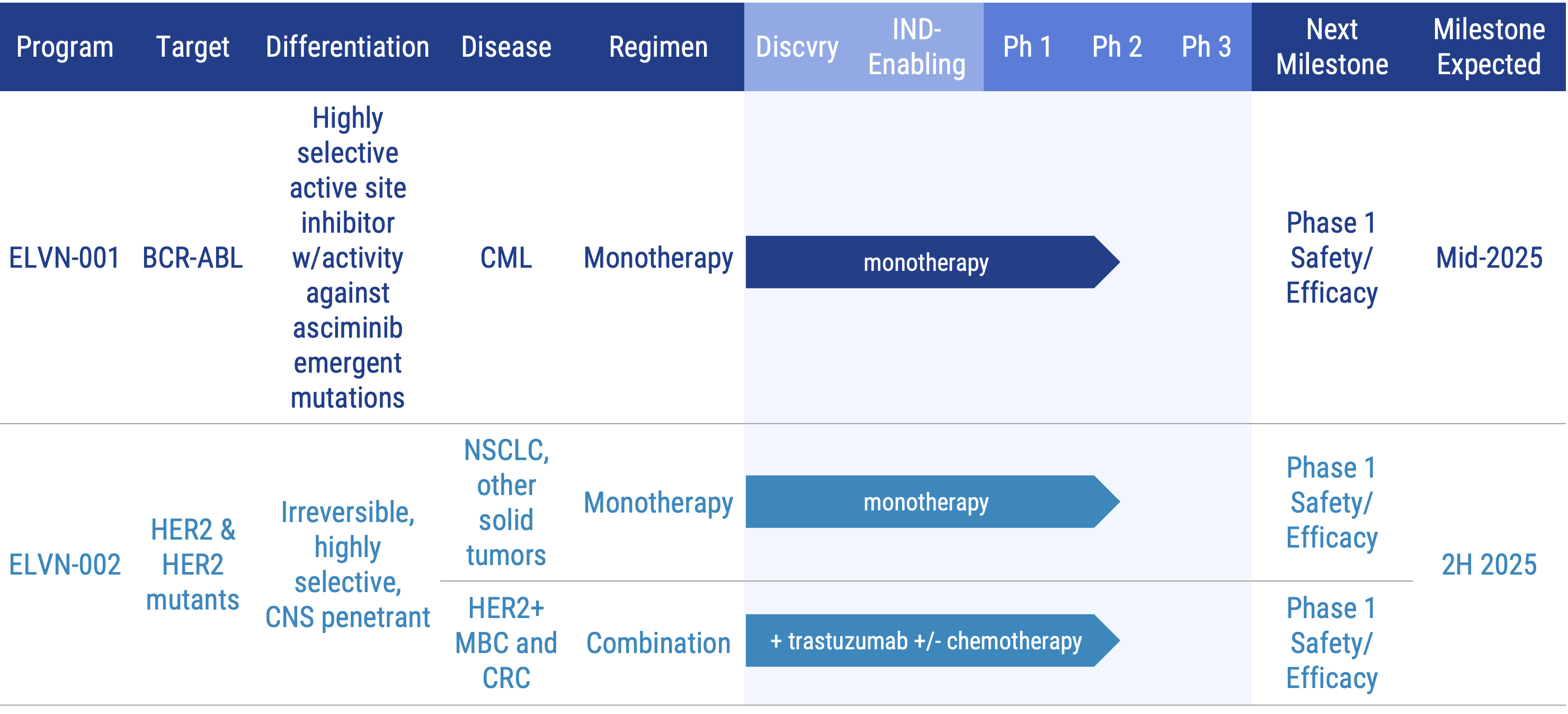


BCR-ABL Program: ELVN-001
ELVN-001, is a potent, highly selective, small molecule kinase inhibitor designed to specifically target the BCR-ABL fusion gene product, the oncogenic driver for patients with chronic myeloid leukemia (CML). ELVN-001 targets the ATP-binding site of the ABL1 kinase domain and binds to a unique P-loop “folded-in” active conformation of ABL1, creating a narrow selectivity tunnel against the broader kinome.
ELVN-001 was also designed to have activity against the T315I mutation, the most common BCR-ABL mutation, which confers resistance to nearly all approved tyrosine kinase inhibitors (TKIs), as well as activity against mutations known to confer resistance to allosteric BCR-ABL inhibitors.
Although the approval of BCR-ABL TKIs has significantly improved the life expectancy of patients with CML, tolerability, safety, resistance and patient convenience concerns have become more prominent as patients can now expect to live on therapy for decades. These issues can result in the loss of molecular response and disease progression for many patients and drive approximately 20% of patients to switch therapy within the first year of therapy and approximately 40% to switch in the first five years of therapy.
As a highly selective active site inhibitor, ELVN-001 has a unique mechanism of action, and therefore potentially represents a complementary option to allosteric BCR-ABL inhibitors, which may play an increasingly important role in the standard of care.
We believe that, given its marked kinome selectivity, attractive drug-like properties, and activity against T315I, ELVN-001 has the potential to represent an improved option for patients with CML across all lines of therapy in the future. Importantly, ELVN-001 was designed to be a more attractive option for patients with comorbidities, on concomitant medications or desiring more freedom from stringent administration requirements.
ELVN-001 is currently being evaluated in the ENABLE study, a Phase 1 clinical trial, in adults with CML. We recently announced updated, positive data from the trial. Based on these results, we are preparing for a potential pivotal trial to begin in 2026. An abstract summarizing the most recent data is available on the Program Presentations & Publications section of the website. To learn more about the ongoing ENABLE study, visit clinicaltrials.gov. (NCT05304377).
Understanding Clinical Benefit in CML
HER2 Program: ELVN-002
ELVN-002, is a potent, highly selective, central nervous system (CNS) penetrant and irreversible HER2 inhibitor with activity against wild type HER2 and various HER2 mutations. The overexpression, amplification or mutation of HER2 is closely associated with aggressive forms of solid cancers, including breast cancer (BRC), non-small cell lung cancer (NSCLC), colorectal cancer (CRC), and several others.
ELVN-002 is designed to inhibit wild-type HER2 and key mutations of HER2, while sparing wild-type EGFR and avoiding EGFR-related toxicities. We believe that if ELVN-002 achieves this profile, it will be able to achieve an improved therapeutic index compared to current approved and investigational TKIs as well as provide a meaningful therapeutic option to patients with brain metastases, a key mechanism of resistance to current therapies in patients with NSCLC, BRC and other HER2 driven cancers. Additionally, ELVN-002 was specifically designed to enable rational combination therapies, which we believe will be important for the treatment of patients with metastatic HER2 overexpressing and amplified cancers.
Due to the recent approvals and success of antibody drug conjugates (ADC) targeting HER2 expressing cancers, the HER2-altered cancer treatment paradigm is shifting. Because of this dynamic and the fact that a standard of care regimen has not been established for patients that progress or are intolerant to this new treatment modality, we believe there is a significant patient need and market opportunity in the post-ADC setting. Based on precedent established by tucatinib in HER2+ BRC and CRC, we believe dual HER2-targeting may represent an important treatment option for patients with HER2+ cancers, especially for those who progress or are intolerant to previously approved treatments.
ELVN-002 is being evaluated in a Phase 1 trial as a monotherapy agent in people with HER2+ and HER2 mutant tumors. As part of that trial, we are also enrolling patients with HER2+ metastatic breast cancer (MBC) in an exploratory cohort in combination with ado-trastuzumab emtansine and patients with HER2+ NSCLC in an exploratory cohort in combination with trastuzumab deruxtecan. To learn more, please visit www.clinicaltrials.gov (NCT05650879).
ELVN-002 is also being evaluated in combination with trastuzumab with or without chemotherapeutic agents in people with HER2+ solid tumors. To learn more about the Phase 1 study, please visit www.clinicaltrials.gov (NCT06328738).
Additional Programs
We believe that our development approach, which is rooted in validated biology and differentiated chemistry, represents a unique opportunity to provide patients with medicines offering improved therapeutic profiles. To capitalize on this opportunity, we continue to support and advance our research pipeline in areas in which we believe we can solve complex medicinal chemistry problems in a target- and indication-agnostic approach.
Program Presentations & Publications
ENABLE: A Phase 1a/1b Study of ELVN-001, a selective active site inhibitor of BCR::ABL1, in patients with previously treated CML
European Hematology Association Congress (oral presentation) | June 2025
ENABLE: A Phase 1a/1b Study of ELVN-001, a selective active site inhibitor of BCR::ABL1, in patients with previously treated CML
European Hematology Association Congress (abstract) | May 2025
Development and application of a mechanistic pharmacokinetic pharmacodynamic (PKPD) model to predict anti-chronic myeloid leukemia (CML) effects of tyrosine kinase inhibitors
American Association for Cancer Research Annual Meeting | April 2025
ELVN-001, a highly selective ATP-competitive ABL1 tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia alone or in combination with asciminib
American Association for Cancer Research Annual Meeting | April 2025
ELVN-002, a potent, selective HER2 inhibitor with a differentiated binding mode conferring the potential for enhanced efficacy in combination with HER2-targeting antibody-drug conjugates
American Association for Cancer Research Annual Meeting | April 2025
ELV-3111, a type 1 pan-RAF inhibitor, that safely combines with MEK inhibitors for enhanced anti-tumor activity in NRAS and BRAF mutant cancers including the most common mechanisms of BRAF inhibitor clinical resistance
American Association for Cancer Research Annual Meeting | April 2025
Mechanism of tumor-selective inhibition of dimeric RAF by a Type 1 RAF inhibitor
American Association for Cancer Research Annual Meeting | April 2025
Application of PKPD Principles to Drive the Discovery and Development of Novel BCR-ABL Tyrosine Kinase Inhibitors
American Conference on Pharmacometrics | November 2024
Preliminary safety and efficacy of ELVN-001, a selective active site inhibitor of BCR::ABL1 in CML
European Society of Hematology International Chronic Myeloid Leukemia Foundation (ESH-iCMLf) 26th Annual John Goldman Conference | September 2024
ELVN-001 Positive Proof of Concept Data from Phase 1 Clinical Trial of ELVN-001 in Chronic Myeloid Leukemia
Company Hosted Event with Key Opinion Leaders | April 2024
Trials in Progress: Phase 1a/b study of ELVN‑002 in solid tumors with HER2 mutations, amplification, or overexpression
World Congress of Lung Cancer | September 2023
Preclinical Activity of ELVN-002: a Potent, Selective, Irreversible and CNS Penetrant HER2 and pan-HER2 Mutant Small-Molecule Inhibitor for the Treatment of HER2-Driven Malignancies
American Association for Cancer Research Annual Meeting | April 2023
ELVN-001, a Next-Generation, ATP-Competitive ABL1 Tyrosine Kinase Inhibitor for the Treatment of Chronic Myeloid Leukemia
64th American Association of Hematology Annual Meeting and Exposition | December 2022
Trial in Progress: First-in-human study of ELVN-001, a highly selective BCR::ABL1 tyrosine kinase inhibitor, in patients with chronic myeloid leukemia who failed previous TKI therapies
24th Annual John Goldman Conference on Chronic Myeloid Leukemia: Biology and Therapy | October 2022,
64th American Association of Hematology Annual Meeting and Exposition | December 2022
